Installing the ChemUnits Add-In adds a new menu under Excel’s ADD-INS tab. Click here to see how to load the ChemUnits Add-In using Mac Excel 2011.

Selecting the ADD-INS tab will show the ChemUnits menu, which contains the four ChemUnits functions. To see the ChemUnits menu in Excel for Mac OS X, click here.
To calculate the molecular mass of a chemical formula, select “Formula Mass” from the ChemUnits menu under the ADD-INS tab. In Mac Excel, select “Formula Mass” from the ChemUnits menu.
This will bring up the Formula Mass dialog box where you can enter your chemical formula. In this example we will calculate the mass of ammonium phosphate, (NH4)3PO4. Notice how no special formatting of the chemical formula is required (except, as always, chemical formulas are case-sensitive).
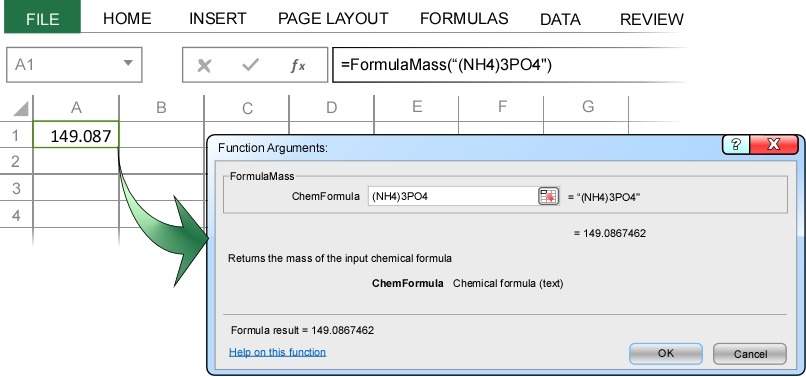
When you press enter, ChemUnits correctly calculates the molecular mass of ammonium phosphate as 149.09 g/mol.
In the example above we typed the chemical formula directly into the Formula Mass dialog box. Just as with any Excel function, we could alternatively enter the address of the cell containing the chemical formula. In the example below, cell A1 contains the chemical formula for calcium carbonate, CaCO3. Highlighting cell B1 and then selecting “Formula Mass” from the ChemUnits menu under the ADD-INS tab opens the Formula Mass dialog box. This time, rather than entering the chemical formula directly, we instead enter the address of the cell containing the formula, cell A1.
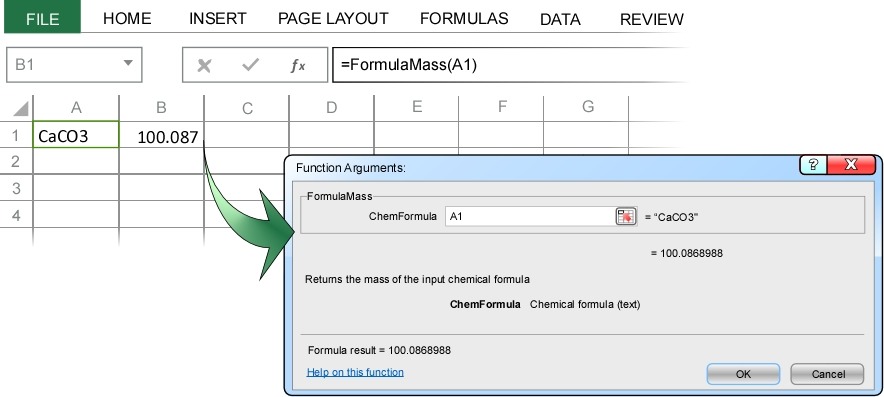
When you press enter, ChemUnits correctly calculates the molecular mass of calcium carbonate as 100.087 g/mol.
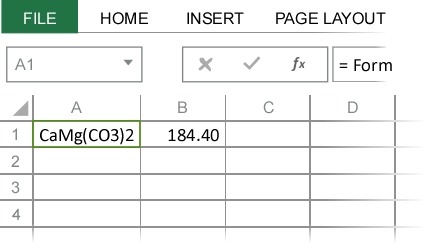
The advantage of referencing cells rather than directly inputting formulas into the dialog box is that, as with any Excel function, the molecular mass is automatically updated whenever the chemical formula is change. For example, if you change the chemical formula in cell A1 to dolomite (CaMg(CO3)2), the molecular mass is automatically updated to the correct value (184.40).
Many different programs and websites can calculate molecular masses, but what sets ChemUnits apart is its ability to convert masses and concentrations. As a first example, consider the common problem of converting between ppm (parts per million) and molarity (moles/liter). Suppose we have a 78 ppm solution of Ca2+. What is the molar (moles/liter) concentration of the calcium solution?
Selecting “Convert Concentration” from the ChemUnits menu under the ADD-INS tab opens the Convert Concentration dialog box. In Mac Excel, select “Convert Concentration” from the ChemUnits menu.

In the Convert Concentration dialog box we enter 78 as the value, ppm as the input units, Ca as the (input) chemical formula, and moles/liter as the output units.
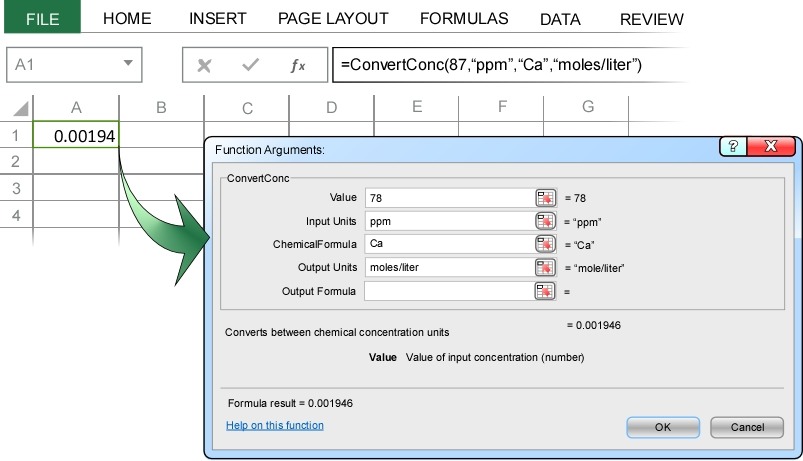
When you press enter, ChemUnits correctly converts 78 ppm Ca2+ to 0.0019 moles/liter. Note that instead of typing “moles/liter” as the output units, you could have equivalently typed “mole/liter”, “mol/l”, or “M”. ChemUnits interprets all these variations as equivalent to molarity.
In the example above we typed the chemical formula directly into the Convert Concentration dialog box. Just as with any Excel function, we could alternatively enter the addresses of the cells containing the required input. Below we solve the same problem as above, except this time referencing the appropriate cells containing the required input:
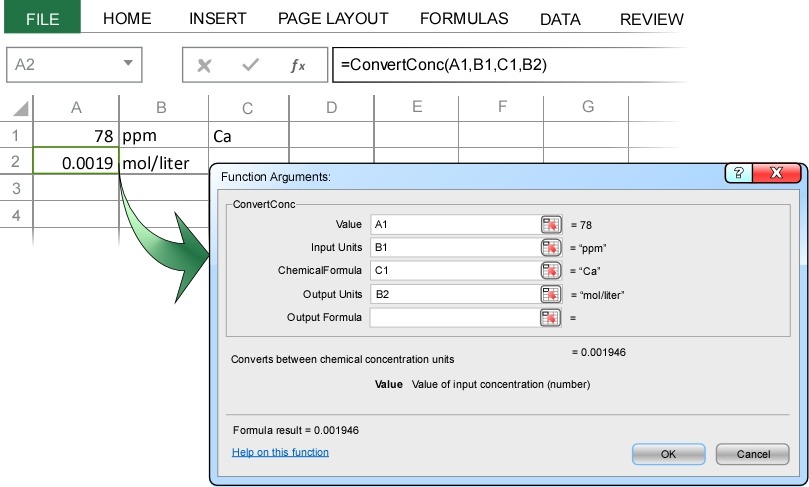
As before, the advantage of referencing cells rather than directly inputting formulas into the dialog box is that, as with any Excel function, the conversion is automatically updated whenever the data change. For example, in addition to “moles”, ChemUnits also recognizes “kilomoles”, “millimoles”, “micromoles”, and “nanomoles” (and all their common abbreviations, e.g. “kmol", “mmol", “umol", nmol”). So in the above example, if you change cell B2 to “mmol/liter”, the converted concentration will automatically update to 1.9 mmol/liter.
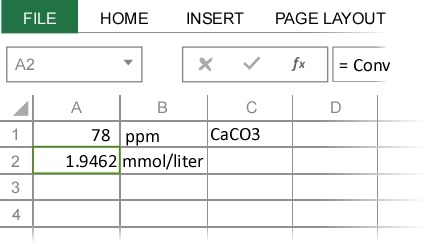
Particularly when dissolutions are involved, we are often interested the resulting concentration of one of the ions. For example, we might want to know the calcium ion concentration in a 0.01 M CaCO3 solution. These types of problems are easily solved using ChemUnits by specifying the output chemical formula in the Convert Concentration dialog.
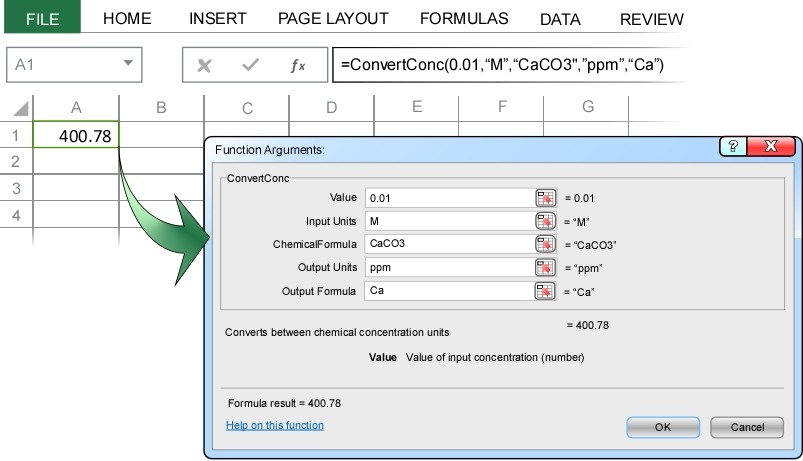
ChemUnits correctly calculates that the calcium ion concentration in a 0.01 M CaCO3 solution in 400.78 ppm.
A common variant of this type of problem arises in making stock solutions. For example, suppose you wanted to make a liter of a 70 mM chloride solution. What mass of magnesium chloride (MgCl2) should be added to each liter of water? To solve this using ChemUnits, we simply convert 70 mM of chloride ion into the equivalent concentration (g/liter) of MgCl2.
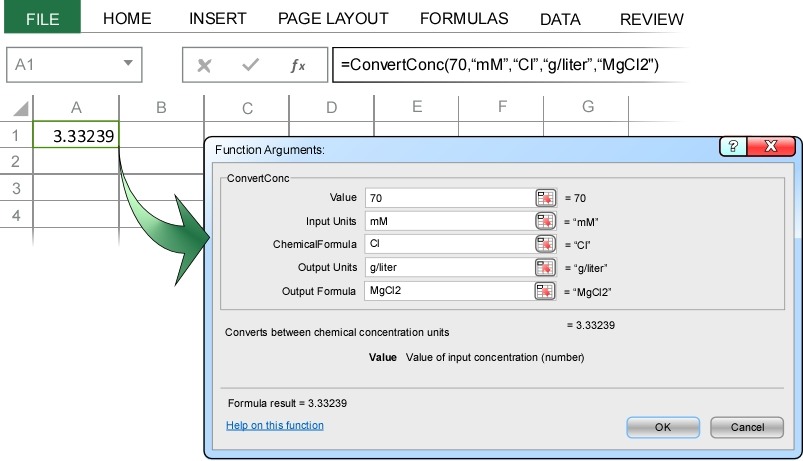
The conversion indicates that we need 3.33 g/liter MgCl2 to make a 70 mM chloride solution.
ChemUnits can convert any combination of units. This can be extremely useful when considering fluxes. For example, typical methane (CH4) fluxes from landfills are about 200 mmol/m^2/day. Using ChemUnits this flux is easily converted to annual total methane emission, for example tons/mile^2/year.
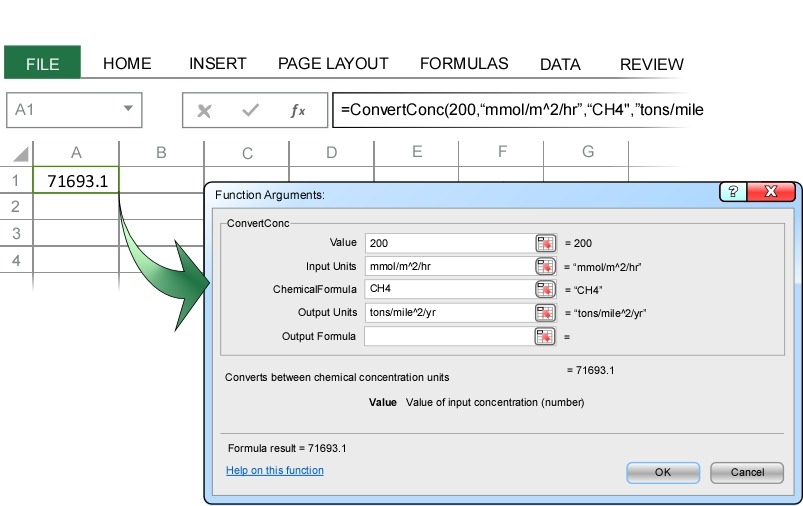
More than 70,000 tons of methane are emitted from each square mile of landfill each year. If we are interested in the carbon cycle and only want to know the total mass of carbon emitted, we just enter carbon as the Output Formula:
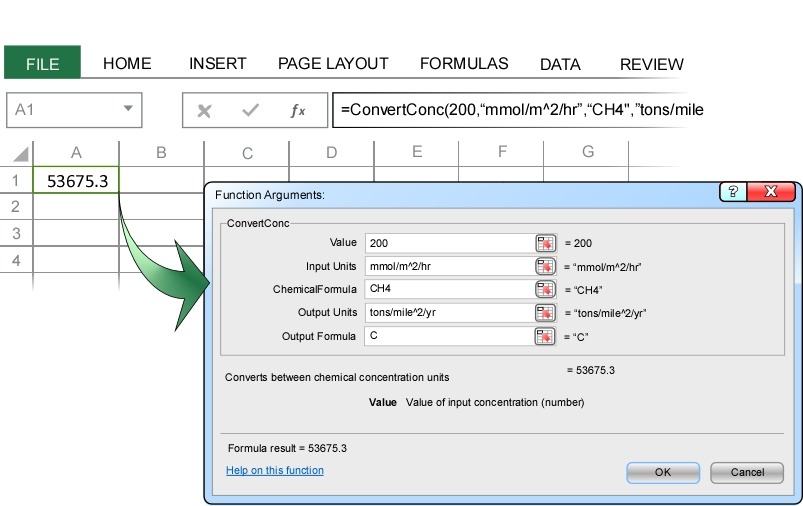
This shows that a methane emission rate of 200 mmol/m^2/hr is equivalent to a carbon emission rate of nearly 54,000 tons/mile^2/year.
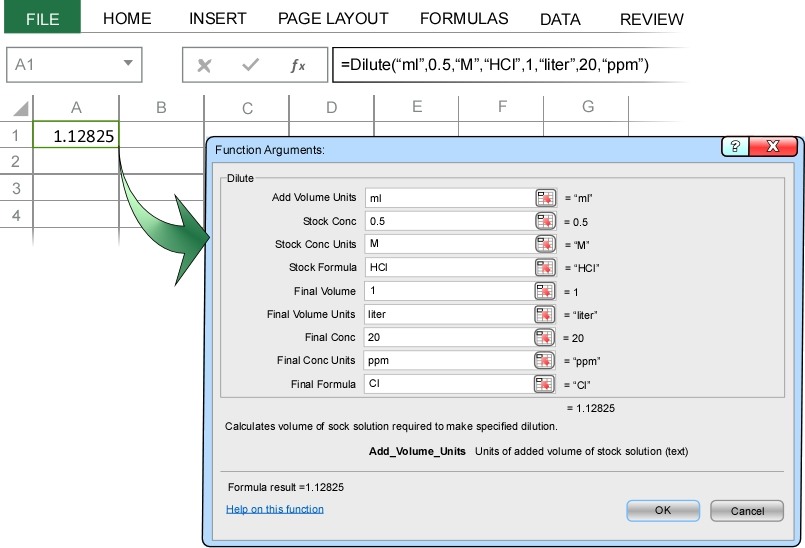
A common task in chemistry is to make a dilute solution from a stock solution. For example, suppose you need to make 1 liter of a solution that is 20 ppm HCl from a stock solution that is 0.5 molar?
Selecting “Dilute Concentration” from the ChemUnits menu under the ADD-INS tab opens the Dilute Concentration dialog box. In Mac Excel, select “Dilute Concentration” from the ChemUnits menu.
In the Dilute Concentration dialog box we enter ml at the units of volume of stock solution to add to the dilution, enter 0.5 M as the stock concentration and stock concentration units, HCl as the chemical formula of the stock solution, 1 liter as the final volume and final volume units of the dilution, and 20 ppm as the desired dilution concentration and dilution concentration units.
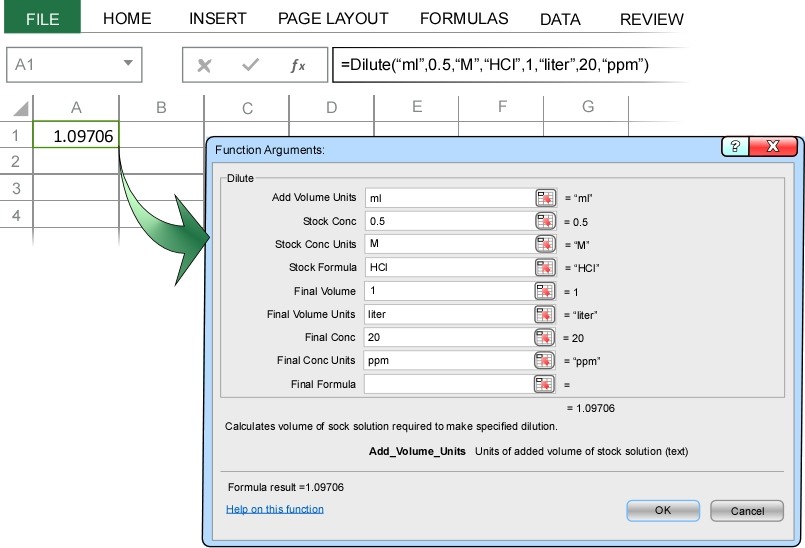
Note: The above image shows all the input, but in Excel only the first 5 input parameters are visible. You will need to scroll down in the dialog box to see the remaining input parameters.
When you press enter, ChemUnits correctly calculates the 1.1 ml of stock solution are required to make 1 liter of 20 ppm HCl solution (the remaining 997.9 ml of the dilute solution are pure water).
A variant of the above example is as follows — suppose you need to make 1 liter of a 20 ppm chloride solution from a stock solution that is 0.5 molar HCl? This type of problem is easily solved by specifying the chemical formula used in defining the concentration of the dilute solution, in this case Cl (rather than HCl).